689 views
Dakshinamurthy et al.: Bronchial Artery Embolisation in Hemoptysis
Review Article
Bronchial Artery Embolisation in Hemoptysis
Dakshinamurthy1, Monisha1, Narasimhan.R2 and Ravi Kumar3
1Postgraduate, 2Senior Consultant, Department of Respiratory Medicine, Apollo Hospitals, Chennai
3Senior Consultant, Department of Interventional Radiology, Apollo Hospitals, Chennai
Corresponding Author: Dr. Antonious Maria Selvam.S, Assistant professor, Sri Manakula Vinayagar Medical College & Hospital, Puducherry
Abstract:
Massive hemoptysis, defined as an expectoration of more than 300 ml of blood in a day,1 has a very high mortality when left untreated2. The management of massive hemoptysis and recurrent non-massive hemoptysis( expectoration of > 100 ml of blood in a day for a few days or weeks) includes surgical and endovascular treatment. Most commonly, these patients suffer from diffuse interstitial lung disease or chronic granulomatous disease such as cystic fibrosis, interstitial pulmonary fibrosis, bronchiectasis, tuberculosis, and fungal infections such as aspergillosis. Less common causes of massive hemoptysis include aneurysms, arteriovenous fistulae, pulmonary embolism, and neoplasms ranging from small benign endobronchial lesions to large malignant tumors.
Endovascular management in bleeding states has established itself as a promising and effective treatment modality. Bronchial artery embolization (BAE) is an ideal first-line treatment because actively bleeding patients with poor underlying pulmonary function tend to be poor surgical candidates. In addition, unlike surgical resection, BAE often preserves pulmonary function. BAE was first described by Remy in 19733. Since then, the procedure has proven its safety and effectiveness in controlling hemoptysis in diverse lung conditions in different studies 4-7.
Corresponding Author: Dr.Dakshinamurthy, Postgraduate, Department of Respiratory Medicine, Apollo Hospitals
How to cite this article: Dakshinamurthy, Monisha, Narasimhan.R and Ravi Kumar ,Bronchial Artery Embolisation in Hemoptysis, JAPT 2019 2[1]:18-21
| Access this article online | |
| Quick Response Code: | Website: www.aptchest.com |
Pathophysiology of Acute Hemoptysis
In most of the conditions causing massive hemoptysis (90%), the bleeding occurs from the bronchial arteries8 rather than pulmonary circulation. Other less common causes of massive hemoptysis include bleeding source from aorta (aortobronchial fistula) or non-bronchial systemic collaterals9-11. In chronic or acute lung conditions, there are obliterative changes in the pulmonary arteriolar level, leading to enlarged bronchial arteries. These high pressure systemic vessels often tend to rupture owing to increased regional blood pressure in an inflamed lung, leading to hemoptysis. The major cause of mortality in massive hemoptysis is asphyxiation due to hemoptysis, not the bleeding itself12.
Anatomy
Bronchial arteries have variable anatomic patterns13. The most consistently present arterial trunk is the right intercosto-bronchial arterial trunk (ICBT), seen in almost 80% individuals. The other patterns, are described by Cauldwell et al 14. Bronchial arteries originate from the descending thoracic aorta, between the level of fifth and sixth thoracic vertebrae, around the origin of the left main bronchus. Bronchial arteries can be distinguished by their branching pattern and their anatomical course along the major bronchi.
In around 30 % of patients, bronchial arterial origin can be aberrant, i.e., outside the normal origin at T5-T6 level15,16. The common sources of aberrant bronchial arteries include the aortic arch, internal mammary artery, subclavian artery, intercostal artery, costocervical trunk, brachiocephalic artery, pericardiophrenic artery, inferior phrenic artery and even the abdominal aorta17. Normal calibre of the bronchial arteries range from 1.5 mm at the origin to 0.5 mm at the point of entry in a bronchopulmonary segment18 The arteries are defined as being enlarged when the diameter measures more than 2 mm in size19.
While performing BAE, the interventional radiologist should be well versed with the anatomy of bronchial arteries and the origin of vertebral and spinal arteries. Two types of arterial supply to the spine are visualized to arise from the intercostals arteries- the radicular and the anterior medullary arteries. The radicular arteries are small branches supplying the ventral and dorsal spinal nerve roots. They are often visualized during BAE and inadvertent embolization of the arteries does not lead to any clinically significant sequelae.4,5,16. The anterior medullary arteries are less often visualized but are of greater concern as they feed the anterior spinal artery. The artery of Adamkiewicz arises at T9 to T12 levels in most cases and reinforces the anterior spinal artery, which is the primary source of spinal cord perfusion20. Inadvertent embolization of the anterior medullary arteries (identified from their ‘hairpin loop’ on angiography) can lead to spinal cord ischemia.
Indications
The common causes of massive hemoptysis requiring BAE in the developing nations include bronchiectasis secondary to tuberculosis or chronic infections, active tuberculosis, chronic bronchitis, lung abscess, pneumonia or aspergilloma21. However, in the western countries BAE is done in cystic fibrosis, lung abscess, tuberculosis, aspergilloma and lung cancer. Uncommon causes of massive hemoptysis treatable by BAE include bronchial adenoma, endobronchial metastases or bronchial artery aneurysm 22,23.
Pre-Procedure Imaging
The preliminary investigations in hemoptysis include plain radiograph, computed tomography (CT) and CT angiography of the chest and bronchoscopy whenever possible. The role of the imaging includes the evaluation of the lungs and localization of the bleeding. CT is helpful in localizing the site of bleed in 63-100% of patients24,25. CT is particularly helpful in cases with tuberculosis, bronchiectasis, bronchogenic carcinoma. Bronchoscopy, once considered as the primary method to localize the bleeding source in hemoptysis, has been shown to have a less chance of localizing the source compared to CECT. In addition to the parenchymal pathology, CECT is also helpful in detecting the enlarged bronchial and nonbronchial arteries25. With the advent of MDCT, detailed CT angiographic evaluation of the thoracic aorta, the bronchial and systemic arteries prior to the procedure is proven helpful. MDCT allows quick evaluation of the lung, airway and the bronchial arteries; whereas fibreoptic bronchoscopy requires sedation and is time-consuming. The current suggestion is to perform CT prior to a bronchoscopy; and the combined use of both the modalities increases the diagnostic yield. MDCT can also evaluate pulmonary vasculature as a cause of hemoptysis.
Angiography Technique and Embolization
Before BAE, a preliminary evaluation of bronchial and nonbronchial arterial supply is essential. For this a thoracic flush aortogram through transfemoral approach is acquired using a 5 F pigtail catheter. This is followed by selective bronchial artery catheterization using a 4F pre-shaped catheter like Celiac, Picard or renal double curve or Simmons. The coaxial microcatheter is also used sometimes. Abnormal angiographic findings include enlargement and tortuosity of the artery, parenchymal blush, shunting to pulmonary artery or vein, aneurysm and contrast extravasation. These abnormal bronchial and nonbronchial arteries are embolized depending upon their supply to the lesion. Careful evaluation of bronchial arterial anatomy is essential to avoid embolization of any spinal artery.
While catheterizing an aberrant bronchial artery originating from the subclavian artery, the origin of vertebral artery should be carefully evaluated to avoid any inadvertent passage of particles in the vertebral artery. Microcatheter is useful in such situation to avoid non-target embolization. Detailed evaluation of the bronchial and nonbronchial systemic arteries is essential. The most common embolizing materials used are gelatine sponge (gelfoam) and polyvinyl alcohol (PVA) particle.
Gelfoam, being a temporary embolizing material, has the disadvantage of recurrence of hemoptysis. PVA particles are permanent embolizing materials. The particle size commonly used in BAE is 300- 500microns26. Use of a smaller sized particle leads to passage of particle through bronchopulmonary anastomosis, pulmonary infarct and nontarget embolization27. The particles are usually mixed with iodinated contrast material in order to make the mixture radio-opaque. The particles are injected manually in small aliquots (1 ml) in a controlled manner under direct fluoroscopic visualization.
Injection should be stopped immediately once there is stasis of the particles to avoid reflux into the aorta and non-target embolization. Compared to gelfoam, PVA particles have less chance of recanalization and hence recurrence of hemoptysis. Absolute alcohol and N-butyl cyanoacrylate glue are other liquid embolizing materials which are not routinely used because of the extensive tissue necrosis associated with them. Metallic coils are not the preferred embolizing agents as they lead to more proximal occlusion and loss of subsequent access to the bleeding vessel.
A
B
C
D

E
| Access this article online | |
| Quick Response Code: | Website: www.aptchest.com |
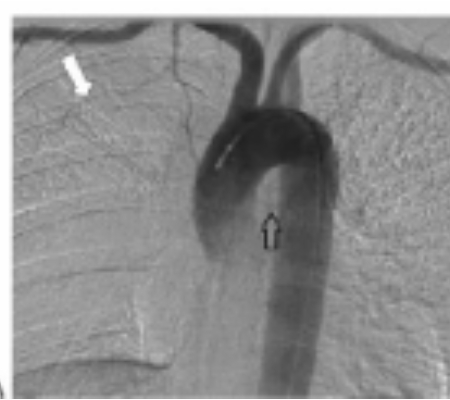
(A) Aortogram, early arterial phase frontal projection, demonstrates a faint bronchial artery superimposed over the middescending thoracic aorta (open black arrow) as well as a prominent branch (white arrow) of the internal mammary artery superimposed over the right upper lobe.
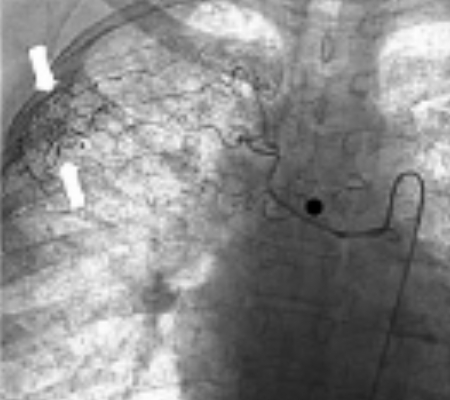
(B) Selective arteriography of the right intercostobronchial trunk shown in Figure 2A demonstrates supply to several intercostals arteries as well as a focus of hypervascularity (arrows) in the right lateral apex. This vessel was embolized with 500 to 700 micron particles injected through a microcatheter.
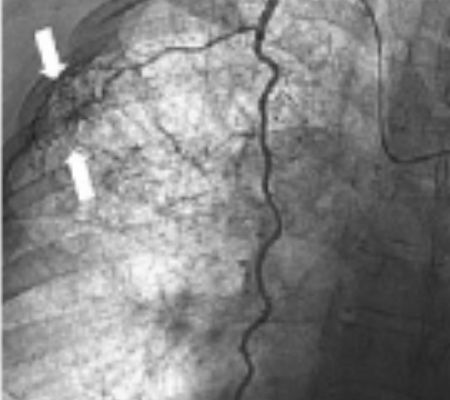
(C) Selective arteriography of the right internal mammary artery demonstrates an enlarged branch (arrows) supplying the same hypervascular region previously embolized.
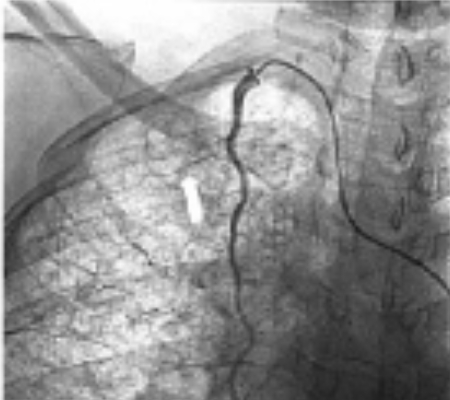
(D) The internal mammary arterial branch was selectively catheterized with a microcatheter (arrow) and embolized with 500 to 700 micron particles.
(E) Selective arteriography of the right internal mammary artery postembolization demonstrates stasis of this branch after embolization
Safety and Efficacy
BAE is commonly used for treating massive hemoptysis; though chronic recurrent mild and recurrent hemoptysis which disturbs lifestyle of the patient and is not amenable to surgical treatment is also increasingly being treated with BAE. BAE is very effective in acute control of hemoptysis (73-98% initial nonrecurrence rates). Superselective catheterization and complete embolization improved the initial success rate. The long term success of this procedure is limited by recurrence of hemoptysis in a significant number of patients (10-52%)28. The recurrence rate depends on several factors including the primary disease condition, adequacy of the procedure and presence of non-bronchial systemic collaterals.
The causes of delayed recurrence includes incomplete embolization, recanalization of the embolized vessel, development of other systemic collaterals and progression of the underlying disease28. In several reports, the major conditions in which recurrence has been noted include tuberculosis, aspergilloma and lung cancer28,29. Neoplasm is an uncommon indication for BAE and is most successful as a temporizing measure prior to surgical resection. Hayakawa et al demonstrated that neoplasm-induced hemoptysis demonstrated the highest failure rate with the worst long-term results. 10 Definitive treatment of the underlying condition is essential to provide cure of the disease. In cases of recurrent bleed, repeat BAE has proven useful.
Complications
Bronchial artery embolization is a safe procedure. Transient chest pain is the most common complication.Subintimal dissection of the bronchial artery or the aorta has been reported in up to 6.3% of patients 4,5, 30-32. Usually this is inconsequential. Non target embolization of the anterior spinal arteries leading to spinal cord ischemia is the most dreaded complication of the procedure. The reported incidence of such complications range from 1.4-6.5%33,34 Dysphagia can occur secondary to embolization of the esophageal branches; which usually resolves spontaneously. Rare complications of BAE include bronchoesophageal fistula, transverse myelitis, bronchial infarction, ischemic colitis and stroke, pulmonary infarction, pericarditis, and transient cortical blindness(possibly due to collateral vessel between the bronchial and vertebral arteries) 35-39.
Conclusion
BAE is a safe and effective treatment modality which can be life saving in massive hemoptysis. In chronic recurrent non massive hemoptysis also its utility has been established. Though long term efficacy of BAE is limited in chronic diseases, it can be repeated in cases of recurrent hemoptysis. Imaging, in the form of MDCT and CT angiography, is useful in the identification of abnormal bronchial and nonbronchial systemic arteries before performing BAE.
References
- Hayakawa K, Tanaka F, Torizuka T, et al. Bronchial artery embolisation for haemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol 1992; 15:154-159.
- Jean-Baptiste, E. Clinical assessment and management of massive haemoptysis. Crit Care Med 2000; 28:1642-1647
- Remy J, Arnaud A, Fardou H, Giraud R, Voisin C. Treatment of hemoptysis by embolization of bronchial arteries. Radiology 1977; 122:33–37.
- Uflacker R, Kaemmerer A, Neves C, Picon PD. Management of massive hemoptysis by bronchialartery embolization. Radiology 1983; 146:627–634.
- Uflacker R, Kaemmerer A, Picon PD, et al. Bronchial artery embolization in the management ofhemoptysis: technical aspects and long-term results. Radiology 1985; 157:637–644.
- Keller FS, Rosch J, Loflin TG, Nath PH, McElvein RB. Nonbronchial systemic collateral arteries:significance in percutaneous embolotherapy for hemoptysis. Radiology 1987; 164:687–692.
- Antonelli M, Midulla F, Tancredi G, Salvatori FM, Bonci E, Cimino G et al. Bronchial artery embolization for the management of nonmassivehemoptysis in cystic fibrosis.Chest 2002;121:796-801.
- Remy J, Remy-Jardin M, Voisin C. Endovascular management of bronchial bleeding. In: Butler J, ed. The bronchial circulation. New York, NY: Dekker, 1992; 667– 723.
- MacIntosh EL, Parrott JCW, Unrhu HW. Fistulas between the aorta and tracheobronchial tree. AnnThoracSurg 1991; 51:515–519.
- Hakanson E, Konstantinov IE, Fransson SG. Management of life-threatening haemoptysis. Br JAnaesth 2001; 88:291– 295.
- Hakanson E, Konstantinov IE, Fransson SG. Management of life-threatening haemoptysis. Br JAnaesth 2001; 88:291– 295.
- Pearse EO, Bryan AJ. Massive hemoptysis 27 years after surgery for coarctation of the aorta. J R Soc Med 2001; 94:640–641.
- Marshall TJ, Jackson JE. Vascular intervention in the thorax: bronchial artery embolization forhemoptysis. EurRadiol 1997; 7:1221–1227.
- Lippert H, Pabst R. Bronchial arteries. In: Lippert H, Pabst R, eds. Arterial variations in man. Munich, Germany: Bergmann Verlag, 1985; 18–19.
- Cauldwell EW, Siekert RG, Lininger RE, Anson BJ. The bronchial arteries: an anatomic study of 105 human cadavers. SurgGynecolObstet 1948; 86:395–412.
- Sancho C, Escalante E, Dominguez J, et al. Embolization of bronchial arteries of anomalous origin. Cardiovasc InterventRadiol 1998; 21:300–304.
- Cohen AM, Doershuk CF, Stern RC. Bronchial artery embolization to control hemoptysis in cysticfibrosis. Radiology 1990; 175:401–405.
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life threatening hemoptysis: a comprehensive review.Radiographics 2002;22:1395-1409.
- Deffenbach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation: small, but avital attribute to the lung. Am Rev Respir Dis 1987; 135: 463–481.
- Furuse M, Saito K, Kunieda E, et al. Bronchial arteries: CT demonstration with arteriographic correlation. Radiology 1987; 162:393–398
- Rosenthal D. Spinal cord ischemia after abdominal aortic
operation: is it preventable? J VascSurg 1999; 30:391–399. - D’Silva L, Muhammed B PM, Mohanty KC. Transcatheter bronchial artery embolization for management of massive hemoptysis. Ind J Tub 1997;44:137-140.
- Suyama H, Igishi T, Makino H, kaminou T, Hashimoto M, Sumikawa T et al. Bronchial arteryembolization before interventional bronchoscopy to avoid uncontrollable bleeding: a case report ofendobronchial metastasis of renal cell carcinoma. Intern Med 2011;50:135-9.
- Quero-Valenzuela F, Piedra-Fernandez I, Sevilla-Lopez S, de Guevara A C. Spontaneous hemomediastinum and hemothorax after dissecting bronchial artery aneurysm. Interactive cardiovascular thoracaicsurg 2011 ( article in press)
- Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177:861– 867.
- Abal AT, Nair PC, Cherian J. Haemoptysis: aetiology, evaluation, and outcome—a prospective study in a third- world country. Respir Med 2001; 95:548–552.
- White RI Jr. Bronchial artery embolotherapy for control of acute hemoptysis: analysis of outcome. Chest 1999; 115:912–915.
- Pump K. Distribution of bronchial arteries in human lung. Chest 1972; 62:447–451.
- Katoh O, Kishikawa T, Yamada H, Matsumoto S, Kudo S. Recurrent bleeding after arterial embolization in patients with hemoptysis. Chest 1990; 97: 541–546.
- Goh PYT, Lin M, Teo N, Wong DES. Embolization for hemoptysis: a six-year review. CardiovascInterventRadiol 2002; 25:17–25.
- Hayakawa K, Tanaka F, Torizuka T, et al. Bronchialartery embolization for hemoptysis: immediate and long-term results. Cardiovasc InterventRadiol1992;15:154–159.
- Mal H, Rullon I, Mellot F, et al. Immediate and longterm results of bronchial artery embolization for life threateninghemoptysis. Chest 1999; 115:996–1001.
- Kato A, Kudo S, Matsumoto K, et al. Bronchial artery embolization for hemoptysis due to benigndiseases: immediate and long-term results. Cardiovasc InterventRadiol 2000; 23:351–357.
- Tanaka N, Yamakado K, Murashima S, et al. Superselective bronchial artery embolization for hemoptysis with a coaxial microcatheter system. J VascInterventRadiol 1997; 8:65–70.
- Wong ML, Szkup P, Hopley MJ. Percutaneous embolotherapy for life-threatening hemoptysis. Chest 2002; 121:95–102.
- Girard P, Baldeyrou P, Lemoine G, Grunewald D. Left mainstem bronchial artery stenosis complicating bronchial artery embolization. Chest 1990; 97:1246–1248.
- Munk PL, Morris C, Nelems B. Left main bronchialesophageal fistula: a complication of bronchialartery embolization. Cardiovasc InterventRadiol 1990; 13: 95–97.
- Remy-Jardin M, Wattinne L, Remy J. Transcatheter occlusion of pulmonary arterial circulation and collateral supply: failures, incidents, and complications. Radiology 1991; 180:699–705.
- Ramankantan R, Ketkar M, Maddali K, Deshmukh H. Referred pain to the ipsilateral forehead and orbit: an unusual phenomenon during bronchial artery embolization. Cardiovasc InterventRadiol 1999; 22: 275– 277.
- Liu SF, Lee TY, Wong SL, Lai YF, Lin AS. Transient cortical blindness: a complication of bronchialartery embolization. Respir Med 1998; 92:983–986.





