663 views
Soofia.M and Arjun.P: Biologicals in Treatment of Asthma
Review Article
Biologicals in Treatment of Asthma
Soofia.M 1 , Arjun P 2
1Senior Registrar, Respiratory Medicine, Kerala Institute of Medical Sciences, Trivandrum – 695029 soofiamohammed2887@gmail.com
2 Sr. Consultant and Group Coordinator, Respiratory Medicine, Kerala Institute of Medical Sciences, Trivandrum – 695029 dr.p.arjun@gmail.com
Corresponding Author: Arjun P, Sr. Consultant and Group Coordinator, Respiratory Medicine, Kerala Institute of Medical Sciences, Trivandrum – 695029. Email: dr.p.arjun@gmail.com
How to cite this article: Soofia.M, Arjun.P, Biologicals in Treatment of Asthma, JAPT 2018; 1:14-19
INTRODUCTION
Asthma is a chronic inflammatory airway disease that is responsible for a large global disease burden. Although effective therapies are available for asthma, the disease in many patients is not adequately controlled. The greatest need in the treatment of asthma; apart from improving adherence, is the development of therapies that would benefit patients who have severe disease despite using current therapies.
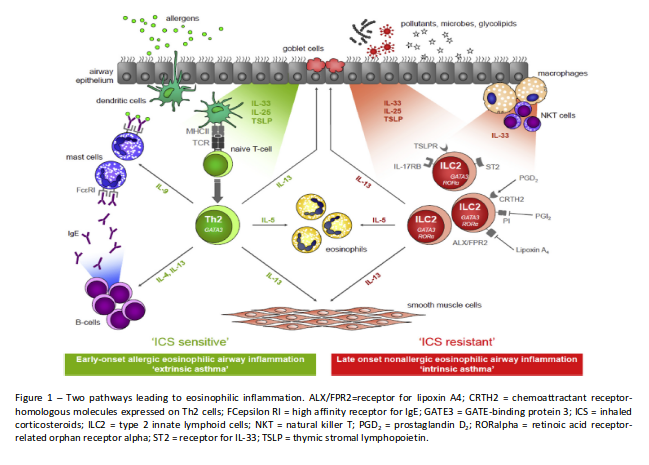
Severe asthma is defined as asthma which requires medications for GINA steps 4–5 asthma Airway epithelial cells exposed to damaging insults such as allergens, viruses, fungi, and pollutants release “alarmin” cytokines (IL-33, IL-25, and thymic stromal lymphopoietin [TSLP]). These alarmins, on the one hand, can initiate an adaptive immune response through dendritic cells that stimulate naïve T cells to differentiate into T helper 2 (Th2) cells. Th2cells produce IL-5, IL-13, and IL-4, the latter driving IgE synthesis by B cells. On the other hand, alarmins can also activate the innate immune system by stimulating type 2 innate lymphoid cells (ILC2), which are also capable of producing large amounts of “Type 2” cytokines (high dose ICS and LABA or leukotriene modifier/theophylline) for the previous year or systemic corticosteroid for > 50% of the previous year to prevent it from becoming ‘uncontrolled’ or which remains ‘uncontrolled’ despite this therapy (1).
Eosinophilic airway inflammation has shown to be one of the most influential traits in chronic airways disease, particularly asthma. Several anti- eosinophilic drugs are now approved for asthma, and there is increasing evidence of beneficial effects in a sub group of patients with COPD as well.
such as IL-5 and IL-13 but not IL-4. The adaptive Th2-mediated pathway has been proposed to be typical for allergic asthma, whereas the ILC2- mediated pathway may be the key mechanism of eosinophilic airway inflammation in nonatopic or “intrinsic” asthma, in which IgE does not seem to play a role (Fig. 1). The relative contribution of these pathways in the different groups of patients with airways disease is not fully clarified.
Biological agents in asthma
ANTI-IgE -Omalizumab
Eosinophilic inflammation can be targetedindirectly by blocking the effects of IgE.
Omalizumab, a monoclonal antibody against IgE, was already approved for allergy-related asthma as early as 2003.
Dose: subcutaneous injection every 2 to 4 wkdepending on dose and dosage is according to body weight and IgE.
Suggested clinical population: Persons with IgE≥30 IU/ml (upper limit of IgE varies according to weight and regulatory authority), positive skin test or elevated specific IgE level in response to perennial aeroallergen; best response is seen in those with FeNO≥20 ppb.
Effect on biomarkers: Small reduction in FeNO, no reduction in circulating total IgE (measured by available assays) Adverse effects: Anaphylaxis (in an estimated 0.2% of patients); monitor for helminthic infection.
GINA 2018 recommendation: for patients aged ≥6 years with moderate orsevere allergic asthma, that is uncontrolled on Step 4 treatment (3).
It has been shown toreduce blood eosinophil counts in patients with severe allergic asthma. In a meta-analysis, reduction in blood eosinophil counts was associated with improved clinical outcomes (4) . A Cochrane review of clinical trials with omalizumab reported reduced unscheduled emergency visits and hospital admissions (5)
New Anti-Eosinophil Drugs
Over the last decade, several monoclonal antibodies and small molecule therapies have been developed for the treatment of eosinophilic or Type 2 airway disease and are now gradually entering the therapeutic armamentarium.
ANTI-IL-5/ANTI-IL-5 Receptor Alpha
Mepolizumab:
Mepolizumab is a humanized monoclonal antibody against circulating IL-5. The antibody neutralizes IL-5 by inhibiting binding of IL-5to the alpha chain of the IL-5 receptor (IL-5Ra) expressed on eosinophils.
Molecular Pathways of Eosinophilic Airway Inflammation (2)
Dose: 100 mg given by monthly subcutaneous injection.
Suggested clinical population: those with two or more exacerbations in past year and ≥300 eosinophils/μl.
Effect on biomarkers: Reduction in circulating eosinophils, no change in FeNO.
Adverse effects: Cases of zoster (rare); avoid in persons with active helminthic infection.
GINA 2018 recommendation: for patients aged ≥12 years with severe eosinophilic asthma that is uncontrolled on Step 4 treatment.
One study with mepolizumab showed a significant 43% reduction in the annual exacerbation rate compared with placebo. Another study showedsafe glucocorticoid-sparing effects in patients with oral steroid-dependent asthma. These promising results were replicated in large Phase III trials, in which 100 mg of mepolizumab was given subcutaneously every 4 weeks (6,7) . The beneficial effects were lost within 6 months after discontinuation of the therapy.
Reslizumab
Reslizumab is another humanized monoclonal antibody that neutralizes IL-5, thereby preventing binding to eosinophils.
Dose: 3 mg/ kg given by monthly intravenous infusion.
Suggested clinical population: Tested primarily in patients with more than one exacerbation in the past year and ≥400 eosinophils/μl
Effect on biomarkers: Reduction in circulating eosinophils, No change in FeNO
Adverse effects: Oropharyngeal pain slightly greater than with placebo, anaphylaxis (rare); avoid in persons with active helminthic infection
GINA 2018 recommendation: for patients aged ≥18 years with severe eosinophilic asthma that is uncontrolled on Step 4 treatment.
In two identical, large Phase III trials, reslizumab 3 mg/kg administered intravenously demonstrated improvements compared with placebo inasthma exacerbations, lung function measures, and quality of life scores (8) .
Benralizumab
Suggested clinical population: Phase 3 efficacy primarily in those with two or more exacerbations in past year and ≥300eosinophils/μl
Benralizumab is a humanized monoclonal antibody that binds to IL-5Ra and rapidly depletes levels of eosinophils and basophils through antibody-dependent cell-mediated cytotoxicity.
Effect on biomarkers: Reduction in circulating eosinophils, no change in FeNO.
In a Phase II b study of patients with severe asthma and high eosinophil counts, 100 mg of benralizumab given subcutaneously every 8 weeks reduced exacerbationrates by 41%, and improved lung function and asthma control. Two recent pivotal Phase III studies- SIROCCO and CALIMA – confirmed these beneficial effects and also showed that benralizumab was well tolerated (9,10).
GINA 2018 recommendation: for patients aged ≥12 years with severe eosinophilic asthma that is uncontrolled on Step 4 treatment.
The ZONDA trial assessed the effect of benralizumab, compared to placebo, on the reduction in the oral glucocorticoid dose while asthma control was maintained in adult patients who had severe asthma there was significant, clinically relevant benefits, as compared with placebo, on oral glucocorticoid use and exacerbation rates (11).
ANTI-IL-13 and ANTI-IL-4Ra
Dupilumab
Dupilumab is a fully human Veloc Immune derived monoclonal antibody that is directed against the alpha subunit of the interleukin-4 receptor, thereby blocking both interleukin-4 and interleukin-13 signalling and hence type 2 inflammation. Since it blocks both IL-4 and IL-13 pathways, dupilumab perhaps is the most effective of all the biologicals available to treat of severe asthma.
Suggested clinical population: Tested primarily in patients with more than one exacerbation in the past 1 or 2 yr and ≥300 eosinophils/μl.
Effect on biomarkers: Temporary increase in eosinophils, reduction in FeNO by approximately 30%.
Adverse effects: Reports of eosinophil counts >5000, may affect metabolism of CYP450 substrates; avoid live vaccines, most likely should avoid in persons with active helminthic infection.
In a Phase IIb study, dupilumab given subcutaneously every 2 weeks was shown to improve lung function and reduced severe exacerbations in patients with uncontrolled persistent asthma, even in patients with relatively low blood eosinophil levels (12).
In a phase 3 trial, LIBERTY ASTHMA VENTURE, the efficacy and safety of dupilumab was assessed as compared with placebo, in reducing the maintenance dose of oral glucocorticoids in patients with glucocorticoid- dependent severe asthma (13) . Dupilumab treatment reduced oral glucocorticoid use while decreasing the rate of severe exacerbations and increasing the FEV1.
Another phase 3 trial, LIBERTY ASTHMA QUEST, assessed the efficacy of dupilumab in patients with uncontrolled moderate-to-severe asthma. Patients treated with dupilumab had significantly lower rates of severe asthma exacerbation than those who received placebo, as well as better lung function and asthma control (14). Greater benefits were seen in patients with higher baseline levels of eosinophils.
Lebrikizumab is a monoclonal antibody against IL- 13,which showed a small improvement in lung functionand a reduction in the rate of exacerbations in patientswith moderately severe uncontrolled asthma in a Phase III trial (15) . Patients with elevated type 2 biomarkers (serum periostin and FeNO) had greater increases in FEV1 than thosewithout.
Tralokinumab, another antibody against IL- 13,decreased rescue b-agonist use and improved FEV1. A large Phase IIb trial in patients with severe asthma with frequent exacerbations failed to reduce the exacerbation rate compared with placebo (16). But patients with high periostin levels treated with tralokinumab reported modestly reduced asthma exacerbations and lung function measures. Phase III trials are ongoing.
OTHER TARGETS
Anti-Chemoattractant Receptor-Homologous Molecules Expressed on Th2 Cells
Chemoattractant receptor-homologous molecules expressed on Th2 cells (CRTH2) are also expressed on eosinophils and mediate the chemotactic response to prostaglandin D2. A variety of small molecule antagonists of CRTH2 have been studied in Phase II trials.
The prostaglandin D2 inhibitor timapiprant showed significant improvements in patients with mild to moderate allergic asthma with an eosinophil dominant form of the disease, and a trial with fevipiprant reported a reduction in sputum eosinophil counts and improved symptoms in patients with persistent eosinophilic asthma (17) . Phase III clinical trials with these compounds are ongoing.
Anti-IL-33, Anti-IL-25, and Anti-TSLP
IL-33, IL-25, and TSLP are a triad of alarmins that are released by airway epithelial cells in response to various damaging environmental stimuli that drive polarization toward eosinophilic inflammation both via the ILC2 and Th2 pathway.
Tezepelumab, a monoclonal antibody blocking TSLP, has been shown to protect againstallergen challenge and to reduce airway eosinophilia in patients with asthma (18) . Other Phase II trials targeting alarmin cytokines are underway.
Inflammasome Inhibitors
There is evidence that the NLRP3 (NOD-like receptor [NLR] family, pyrin domain–containing protein 3) inflammasome is activated in severe asthma and COPD, particularly during exacerbations, and selective small-molecule NLRP3 inhibitors, such as cytokine release inhibitory drug (CRID)-3, have now been discovered (19) .
Inflammasomes are protein complexes that mediate the coordinated expression of proinflammatory cytokines IL-1 and IL-18 through the regulation of Caspase-1, which releases the active cytokine from inactive precursors.
CONCLUSION
The future of asthma therapy looks very exciting with the advent of the newer biological agents which pave the way for development of personalised medicine or precision medicine or phenotype based treatment in respiratory medicine. These drugs which target specific inflammatory pathways, ensure a precise way of treating the particular phenotypes and also help reduce the unwanted adverse effects of the non phenotypic add on drugs, which we currently use to treat difficult to control and severe asthma.
REFERENCES
- International ERS/ATS guidelines on definition, evaluation Maspero et al Efficacy and Safety of Dupilumab in and treatment of severe asthma 2014.
- Elisabeth H. Bel, Anneke ten Brinke. New Anti-Eosinophil Drugs for Asthma and COPD Targeting the Trait! Chest 2017; 152(6):1276-1282.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2018
- Massanari M, Holgate ST, Busse WW, Jimenez P, Kianifard F, Zeldin R. Effect of omalizumab on peripheral blood eosinophilia inallergic asthma. Respir Med. 2010; 104(2):188-196.
- Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev.2014;(1):CD003559.
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651-659.
- Ortega HG, Liu MC, Pavord ID, et al; MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma.
- N Engl J Med. 2014;371(13):1198-1207.
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophilcounts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, Phase 3 trials. Lancet Respir Med.2015;3(5):355-366.
- Bleecker ER, FitzGerald JM, Chanez P, et al; SIROCCO study investigators. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroidsand long-acting b(2)-agonists (SIROCCO): a randomised,multicentre, placebo-controlled Phase 3 trial. Lancet.2016;388 (10056):2115-2127.
- Fitz Gerald JM, Bleecker ER, Nair P, et al; CALIMA study investigators. Benralizumab, an anti-interleukin-5 receptor amonoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised,double-blind, placebo-controlled Phase 3 trial. Lancet.2016;388 (10056):2128-2141.
- Parameswaran Nair, Sally Wenzel,, Klaus F. Rabe, Arnaud Bourdin, et al Oral Glucocorticoid–Sparing Effect of Benralizumab in Severe Asthma
- N Engl J Med 2017; 376:2448-58.
- Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety inadults with uncontrolled persistent asthma despite use of mediumto-high-dose inhaled corticosteroids plus a long-acting b2 agonist: arandomised double-blind placebo- controlled pivotal Phase 2b doserangingtrial. Lancet. 2016; 388(10039):31-44.
- Klaus F. Rabe, Parameswaran Nair, Guy Brusselle, Jorge F. Maspero et al Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma N Engl J Med 2018;378:2475-85.
- M. Castro, J. Corren, I.D. Pavord, J. Masperoet al Dupilumab Efficacy and Safety in Moderate to-Severe Uncontrolled Asthma N Engl J Med 2018; 378:2486-96.
- Hanania NA, Noonan M, Corren J, et al. Lebrikizumab in moderate to-severe asthma: pooled data from two randomised placebocontrolledstudies. Thorax 2015;70(8):748-756.
- Brightling CE, Chanez P, Leigh R, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: arandomised, double-blind, placebo-controlled, Phase 2b trial. Lancet
- Respir Med. 2015;3(9):692-701.
- Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group.
- placebo- controlled trial. Lancet Respir Med. 2016;4(9):699-707.
- Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med.
- 2014;370(22):2102-2110.
- Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz- Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, et al. Asmall-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015;21: 248–255.





