656 views
Jayakumar,S et al.: Role of Bronchial Thermoplasty in Asthma
Review Article
Role of Bronchial Thermoplasty in Asthma
Jayakumar.S 1 , Winnie Elizabeth Jose 2 ,Narasimhan.R 3
1Postgraduate, 2 Professor, 3 Senior Consultant, Department of Respiratory Medicine. Apollo Main Hospitals, Chennai
ABSTRACT:
Bronchial thermoplasty is a novel treatment for patients with severe asthma who continue to be symptomatic despite medical treatment.It aims at reducing smooth muscle mass in the airways by delivering controlled thermal energy to the airway walls during a series of three bronchoscopies. Randomizedtrials of bronchial thermoplasty in severe asthma have not been able to show a reduction in airway hyper responsiveness or change in FEV1 but have suggested an improvement in quality of life, as well as reduction in rate of severe exacerbation, emergency department visits and days lost from work .Strict inclusion and exclusion criteria of these trials resulted in elimination of patients with severe asthma who experienced more than three exacerbation per year. Therefore the generalizability of this treatment to the severe asthma population still needs to be determined. The short term adverse events consists primarily of airway inflammation and occasionally more severe events requiring hospitalization. Long term safety data are evolving and have shown thus far clinical and functional stability up to 5 years after bronchial thermoplasty treatment. Additional studies on bronchial thermoplasty are needed to establish accurate phenotyping of positive responders, durability of effect, long term safety.
Corresponding Author: Dr. Narasimhan.R, Senior Consultant Department of Respiratory Medicine. Email: drrnarasimhan@gmail.com
How to cite this article: Jayakumar.S, Winnie Elizabeth Jose and Narasimhan.R, Role of Bronchial Thermoplasty in Asthma, JAPT 2018; 1:20-23
INTRODUCTION
Asthma is a chronic inflammatory condition of airways characterized by episodic symptoms of
breathlessness, cough and wheezing, which can wax and wane over time. Approximately , 8.2% of general population is affected. In this 5-10% have severe refractory disease and asthma accounts nearly for half a million hospitalization every year. The pathophysiology of asthma include both chronic airway inflammation and Bronchoconstriction. The Bronchoconstriction can be reversed temporarily with Bronchodilators. But, no long lasting therapy to reduce it has been available till now. Bronchial thermoplasty targets this gap in asthma management.
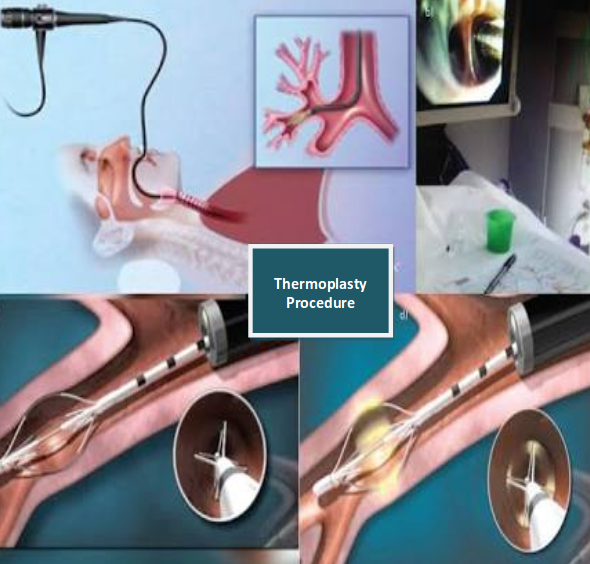
Application of the radio frequency energy to the airways distal to the main stem bronchi down to the airways as small as 3mm in diameter. The treatment is done in 3 separate sessions with careful monitoring before and after for respiratory complication that can occur in severe asthma. Airway complication and asthma exacerbation can occur up to 6 weeks after the last procedure. Bronchial thermoplasty does not cure or completely eliminate symptoms, but it reduces the severity, improves quality of life and decreased emergency visits.
CLAIM
To determine the efficacy and safety of bronchial thermoplasty in adults with bronchial asthma.
EVIDENCE
AIR Trial: The first large multicenter trial of bronchial thermoplasty was prospective and randomized but not blinded. The patients enrolled were 18-65 year old, had moderate or persistent asthma and were requiringinhaled corticosteroids (Beclomethasone 200mcg or more or an equivalent drug) and a long acting beta agonist (100mcg Salmeterol or more or an equivalent drug). They also needed to have FEV1 values 60%-80% of predicted and should have stable asthma for 6 weeks. 112 patients were randomized to receive bronchial thermoplasty with medical therapy or medical therapy alone. Treatment was done in 3 sessions over 9 weeks, followed by attempts to discontinue the LABA at 3, 6, and 9 months after the procedures. Number of mild exacerbation per week was significantly lower at 3 and 12 months in the thermoplasty group with 10 few milder exacerbations per patient per year but was unchanged in the control group. Symptom course, AQLQ were significantly better at 3 and 12 months in the thermoplasty group. Therefore this trial found that thermoplasty improved asthma symptoms within 3 months and the effect lasted 1 year with an encouraging reduction in the number of mild asthma exacerbation. However it was not blinded and there is a strong placebo effect in asthma.
RISA Trial: The inclusion criteria were patients on high dose of inhaled corticosteroids (>750mcg of Fluticasone or its equivalent), taking prednisolone <30mg//day, an FEV1 of at least 50% of predicted
without a bronchodilator, or a positive Methacholine test. 17 patients were randomized to receive bronchial thermoplasty and another 17 patients were randomized to receive medical treatment. Serious adverse events occurred more often in patients with severe asthma in the treatment group than in control group. However 1 year after the procedure the adverse events rates were similar in the treatment and control group suggesting that this procedure can be safely performed in similar patient population.
AIR2 Trial: The major difference in its design compared with the earlier ones was that the control group underwent sham thermoplasty making the trial double blinded study. The thermoplasty group had significantly higher AQLQ scores at 6, 9, 12 months than at baseline.
To summarize this clinical trial showed that bronchial thermoplasty is a feasible, relatively safe procedure producing better clinical outcomes in patients with severe asthma, uncontrolled with medical therapies.
DISCUSSION
Bronchial thermoplasty is an interventional Bronchoscopic procedure for the treatment of severe uncontrolled asthma patients. The treating facility and his/her clinical course should be familiar to the patients in order to ensure appropriate patient selection and optimal asthma control prior to bronchial thermoplasty and during entire treatment period. There should be post bronchodilator FEV1 is <15%, no evidence of asthma exacerbation or acute infection. 50mg of Wysolone was given 3 days before the procedure and on the day of procedure. It is done under conscious sedation and topical Lignocaine. Bronchoscopy inserted, bronchial thermoplasty performed with Aliar(which delivers specific amount of radiofrequency energy through a dedicated catheter. Catheter diploid through 2mm of bronchoscope starting in distal airway as small as 3mm and working proximally, sequentially treat all airways to main stem bronchi. Sites treated are meticulously recorded on a bronchial airway map to avoid skipping or overlapping. In the catheter there are 4 electrodes each electrode has 5mm of exposed wire. Each procedure requires 50-75 activation of the device, on average 44 for right lower lobe, 47 for left lower lobe and 60 for upper lobes. After procedure patient observed for 3-4 hours.
Regarding safety of bronchial thermoplasty 2- 5 follow up results of all 3 major trials are available. There was no increase in asthma symptoms, exacerbation, emergency hospital visits, fall in FEV1 or any other significant adverse events observed in bronchial thermoplasty patients on follow up.
The Global Initiative for Asthma (GINA) now includes Bronchial Thermoplasty as a preferred add-on therapy option for step 5 to help who are still symptomatic on ICS and LABA.
A Cochrane review of the trails found only modest clinical benefit in quality of life and lower rates of asthma exacerbations, but no significant difference in asthma control scores. Further this studies excluded patients who had refractory and relatively more severe asthma. Therefore the results of this trials cannot be extrapolated to this subset of patients for whom this costly treatment is being marketed. Also bronchial thermoplasty can itself lead to increased risk of exacerbation.
CONCLUSION
Bronchial thermoplasty is a new therapeutic bronchoscopic procedure which has been used in research trials in cases of uncontrolled severe asthma in addition to pharmacological therapy. Bronchial thermoplasty causes statistically significant improvement in quality of life decreases future exacerbations and emergency hospital visits. But clinical utility of this improvement in asthma control is little. It does not improve the FEV1. However no significant adverse effects was found in 5 year follow up bronchial thermoplasty patients.
REFERENCES
- Pavord I, Laviolette M, Thomson N et al. 5-year safety of bronchial thermoplasty demonstrated in patients with severe refractory asthma: Research in Severe Asthma (RISA) trial. Am J Respir Crit Care Med 2011; 183: A6382.
- Thomson NC, Rubin AS, Niven RM et al. Long term (5-year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulmonary Medicine 2011; 11:8.
- Castro M, Rubin AS, Laviolette M et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicentric, randomized, double-blind, sham controlled clinical trial. Am J Respir Crit Care Med 2010; 181: 116-24.





