690 views
Journal of the Association of Pulmonologists of Tamil Nadu. Vol. 1, Issue 1, September – December 2018
EDITORIAL
The Surgeon and Pulmonary Tuberculosis: Waxing and Waning Relationship
The birth of thoracic surgery was due to tuberculosis. The first thoracic procedure to be ever done was probably an open drainage of a pleural empyema performed by Hippocrates. Jacobeus in the first decade of 1900s also introduced thoracoscopy for the first time to take down adhesions and pleural biopsy for tuberculosis. A Roman physician by name Gorgio in 1696 reported that a TB patient improved dramatically after sustaining a sword wound to his chest which caused a pneumothorax. This led to the concept of collapse theory for tuberculosis, Carson in 1822 suggested that collapse should be achieved by means of an external force for the affected lung to rest. Forlanini, an Italian physician observed that lung collapse did have a favourable impact on the disease. During the first half of the last century, the discovery of the fact that Mycobacterium tuberculosis was an obligate aerobe led to a rapid proliferation of thoracic surgical procedures for tuberculosis : such as thoracoplasty, artificial pneumothorax, plombage and crushing of the phrenic nerve. The surgeons were basking in the glory of treating tuberculosis.
Waksman and Schatz in 1944 isolated Streptomycin an aminoglycoside antibiotic and tested it on the tuberculosis bacilli. This revolutionized the management of tuberculosis and became the first drug in the medical management of tuberculosis. This landmark event reduced the usefulness of surgeons in management of the disease. In the second half of the 20th century drugs like rifampicin and other anti tuberculous drugs were developed. These wonder drugs created a paradigm shift in the management of tuberculosis leaving very little place for surgeons.
However we are witnessing the resurgence of the role of surgery in the management of tuberculosis. There is an overall increase in the global incidence, the emergence of multidrug resistant TB (resistance to both Rifampicin and INH), extensively drug resistant TB (resistance to Rifampin and Isoniazid, to fluoroquinolones and at least one of the following injectable anti-TB drugs: Capreomycin, Kanamycin, or Amikacin).
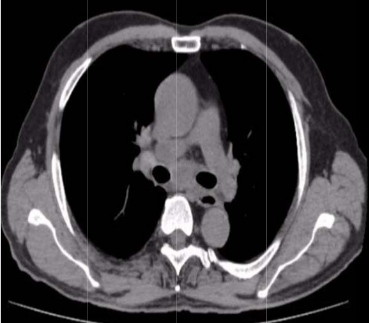
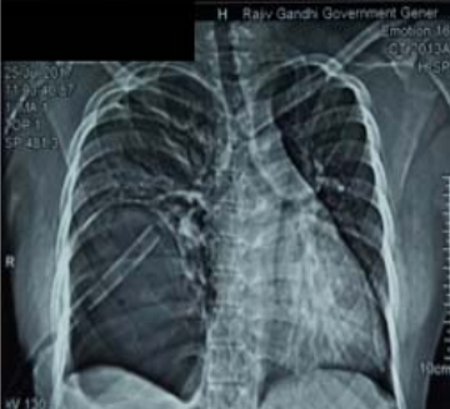
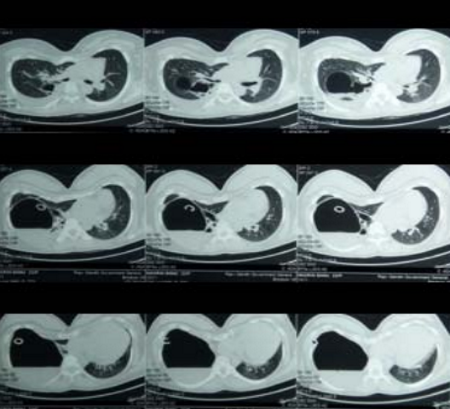
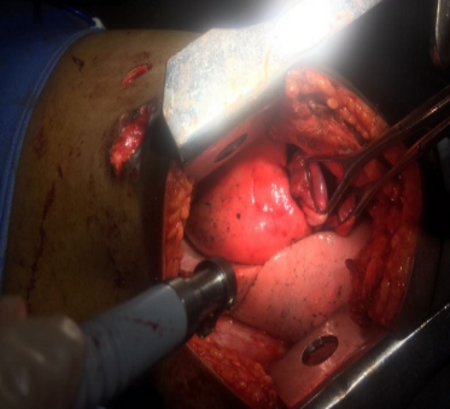
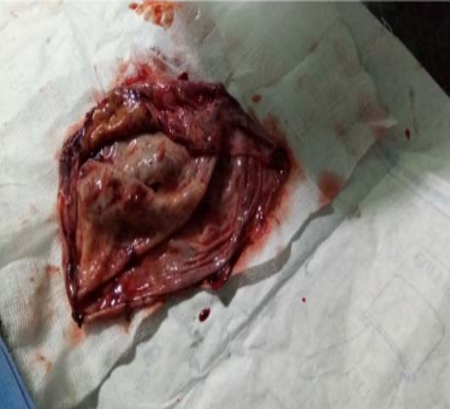
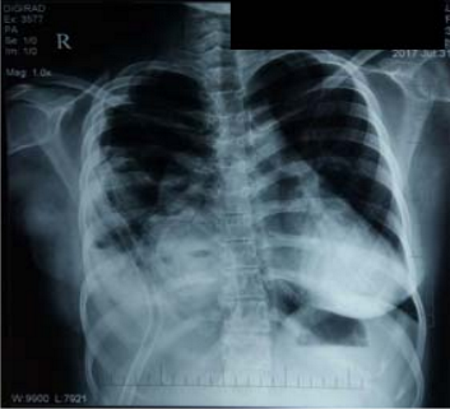
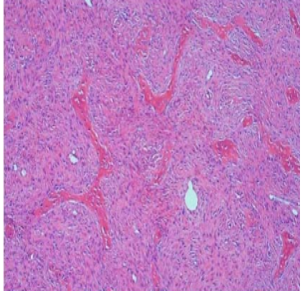
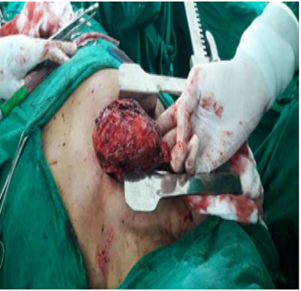
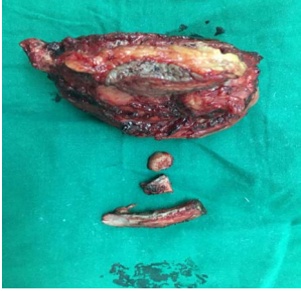
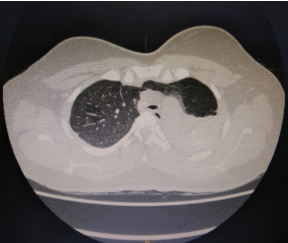
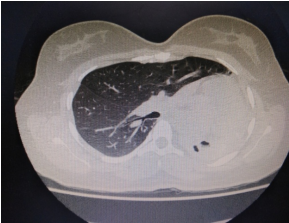
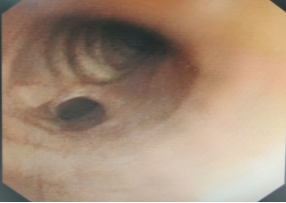
The indications for surgery are the complications and sequelae for tuberculosis. Hemoptysis, destroyed or bronchiectatic lungs, empyema with or without bronchopleural fistula are some of the most common conditions with which patients are referred for surgery.
How to cite this article: Ajay Narasimhan., Editorial, JAPT 2018; 2:47-48
Multi drug resistant tuberculosis is a complex medical entity which forms an important
indication for surgery in a specialised subset of patients. These situations include localised disease, adequate trial of ATT has been given, drug failure and patients are chronically symptomatic.
Surgery for patients with tuberculosis is more difficult than it appears to be. It is of utmost importance that the patient and his relatives are counselled thoroughly regarding the nature of disease and outcome after surgery. They should also be informed about the natural course of disease without any surgical intervention. Pre operative pulmonary rehabilitation plays a major role in improving the pre operative physical fitness of the patient. This includes aggressive chest physiotherapy, postural drainage, nebulisers and antibiotics if necessary. Most of the patients with tuberculosis are nutritionally depleted. Nutritional supplementation plays a major role in improving the post operative outcomes after surgery and reduces the risk of complications such as bronchopleural fistulae and prolonged air leaks.
The outcomes after TB surgery are improved if great detail is given in the post operative period.This includes wound care, care of chest tubes and post operative pulmonary exercises. The common complications after surgery for tuberculosis may be divided into surgical and systemic. The surgical complications include bronchopleural fistula, prolonged air leaks, bleeding and post resectional spaces and empyemas. The medical complications may include respiratory failure, chronic malnutrition.
The advent of minimally invasive thoracic surgical techniques has brought about a renewed zeal in the field of thoracic surgery for tuberculosis. A multidisciplinary team approach involving the pulmonologist, thoracic surgeon, interventional radiologist and infectious disease specialists must work together to actively identify TB patients who are likely to benefit from surgery.