694 views
Amal Johnson et al.: Ubiquitous Fungus Irking Inclined Wheezers – A succinct on Allergic Bronchopulmonary Aspergillosis
Ubiquitous Fungus Irking Inclined Wheezers – A succinct on Allergic Bronchopulmonary Aspergillosis
Amal Johnson1, Vaseema Thabassum2, Narasimhan R3
1Post graduate –Department of Respiratory Medicine, Apollo Hospitals, Greams Road, Chennai, India
2Post graduate –Department of Respiratory Medicine, Apollo Hospitals, Greams Road, Chennai, India
3Senior Consultant Pulmonologist, Apollo Hospitals, Greams Road, Chennai, India
Abstract
Aspergillus mold cause respiratory disease depending upon the host immunity and organism virulence. ABPA is the most significant manifestation of allergic aspergillosis. It is an immunological pulmonary disease caused by hypersensitivity reaction to the aspergillus antigen in susceptible patients, most commonly with asthma and cystic fibrosis. The presentation can range from mild symptoms to extensive lung disease that may manifest as respiratory failure. This review summarizes the history, epidemiology, risk factors, immunopathogenesis, pathology, clinical features, diagnostic criteria, laboratory and radiological investigations and management of ABPA.
Keywords: ABPA, Asthma, Aspergillus, IgE
Corresponding Author: Dr.Amal Johnson, Post graduate, Department of Respiratory Medicine, Apollo Hospitals, Greams Road, Chennai,
India
How to cite this article: Amal Johnson, Vaseema Thabassum, Narasimhan R, “Ubiquitous Fungus Irking Inclined Wheezers” A succinct on Allergic Bronchopulmonary Aspergillosis, JAPT 2019: 2(2):61-67
Introduction
Aspergillus mold cause respiratory disease depending upon the host immunity and organism virulence. The manifestations can be classified into 3 distinct clinical categories; allergic aspergillosis (Allergic Aspergillus sinusitis, ABPA), saprophytic colonization (Aspergilloma) and invasive aspergillosis (Airway invasive aspergillosis, chronic necrotizing aspergillosis and invasive aspergillosis)1. ABPA, the most significant manifestation of allergic aspergillosis is immunological pulmonary disease caused by hypersensitivity reaction (Type 1,3, 4) to the aspergillus fumigatus antigen(Af) in susceptible patients mostly commonly with asthma and cystic fibrosis2,3(Cf).
History of ABPA
ABPA was first described in 1952 by Hinson, Moon and Plummer described three patients with recurrent wheezing, pulmonary infiltrates, eosinophilia in blood and sputum, and brown plugs or flecks in expectorated mucus4.
Epidemiology of ABPA
The prevalence of ABPA in asthma varied between 2.5% and 22.3% according to the International Society for Human and Animal Mycology (ISHAM) working group. Most cases present in the 3rd to 5th decade5.
Genetic Factors Involved in ABPA
Genetic risk factors include expression of HLA-DR2 and HLA-DR5 genotypes, while HLADQ2 protected against ABPA6,7. In subjects with CF, increased chances of Aspergillus colonization of the airways and subsequent development of ABPA were found in those with CF transmembrane conductance regulator gene mutations8,9. Surfactant protein-A2 polymorphisms, elevated levels of mannan-binding lectin due to the 1011A allele, and toll-like receptor polymorphisms 43, IL -4,IL-10,1L13,1L-15 polymorphisms also play an important role in the development of ABPA10,11,12.
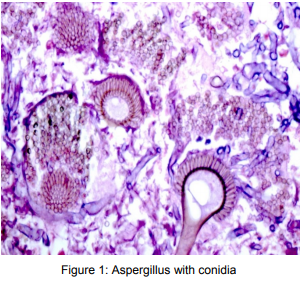
Pathology of ABPA
Histologic examination reveals the presence of mucus, fibrin, Curschmann spirals, CharcotLeyden crystals, and inflammatory cells with scanty hyphae. The bronchial wall in ABPA is usually infiltrated by eosinophils and other inflammatory cells. Bronchocentric granulomatosis, the presence of noncaseating granulomas containing eosinophils and multinucleated giant cells centered on the airway is also a feature14, 15, 16.
Pathogenesis of ABPA
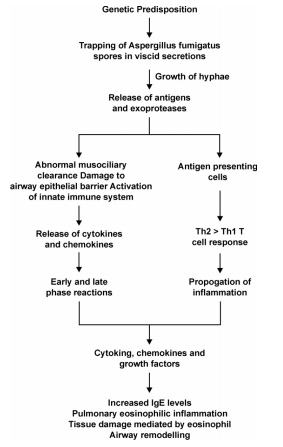
Clinical Features of ABPA
The presentation can range from mild asthma, with very few symptoms, to extensive lung disease that may manifest as respiratory failure. Patients encounter frequent exacerbations and if left untreated, lead to chronic fibrotic lung disease. Respiratory symptoms included cough (99%), breathlessness (99%), expectoration (98%), wheezing (97%), and haemoptysis (41%). Nasal symptoms suggestive of upper airways allergy were present in 45%. Expectoration of sputum plugs was reported by 37% of the patients and nasal plugs by 6%. Approximately half of the patients had a personal/family history of atopy.
Physical examination in ABPA may reveal rhonchi and crepitations. These patients may also exhibit cyanosis, digital clubbing, and features of corpulmonale in end stage fibrotic stages(15%)17.
Diagnostic Criteria
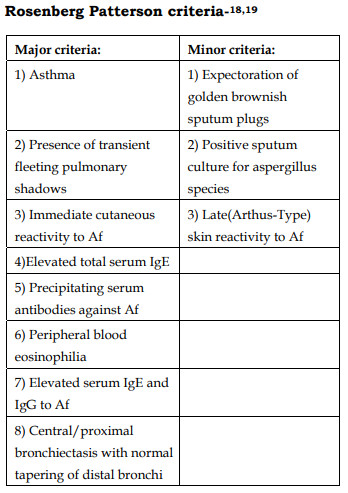
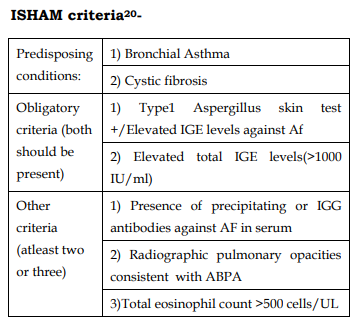
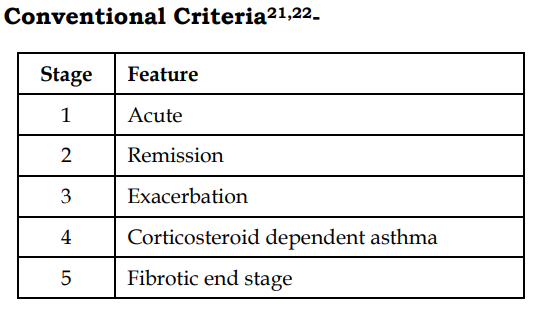
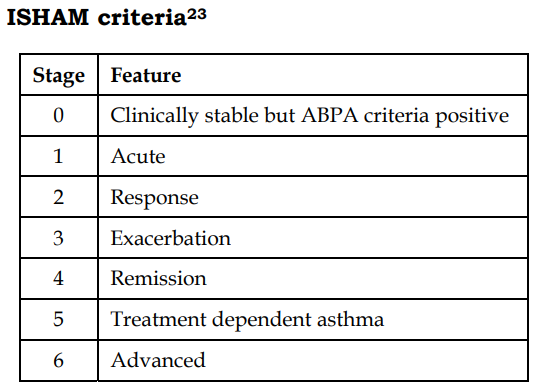
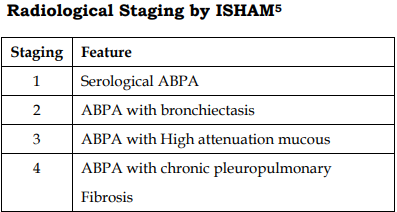
Staging of ABPA:
Investigations
1) Serum Eosinophil Count
Absolute eosinophil count greater than 1000 cells/ul is one of the major criteria for ABPA diagnosis. However, low eosinophil counts does not rule out ABPA24.
2) Skin Testing with Aspergillus Antigens
Aspergillus skin testing is done using commercial or locally prepared Aspergillus fumigatus antigen. The test is read every 15 minutes for 1 hour and then after 6-8 hours. The reactions are classified as Type 1: If wheal and erythema develops within 1 min, reaches maximum within 10-20 minutes and resolves within 1-2 hours and Type 3: Is read after 6 hours and if edema present, termed as positive17.
3) Sputum Cultures for A fumigatus
Culture of A. fumigatus in the sputum is supportive but not diagnostic of ABPA. The fungus can also be grown in patients with other pulmonary diseases due to the ubiquitous nature of the fungi17.
4) Total Serum IgE
The total IgE level is the most useful test for diagnosis and follow-up of ABPA. A normal serum IgE level excludes ABPA as the cause of the patient’s current symptoms. After treatment with glucocorticoids, the serum IgE levels decline, and a >35 % is taken as a criteria for remission. The serum IgE determination is also used for follow-up, and a doubling of the patient’s baseline IgE levels indicates relapse of ABPA25
5) Pulmonary Function Tests
These tests help categorize the severity of the lung disease but have no diagnostic value in ABPA and need not constitute the basis for screening. The usual finding is an mixed obstructive with restrictive defect of varying severity26-29. Normalisation of the parameters can be noticed after treatment.
6) Precipitating Antibodies against A. fumigatus
By the double gel diffusion technique, precipitating antibodies against Af could be detected in the serum upto 92% of patients in concentrated serum30,31.
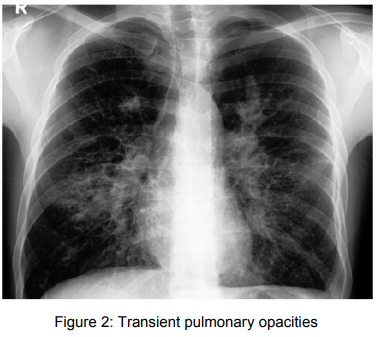
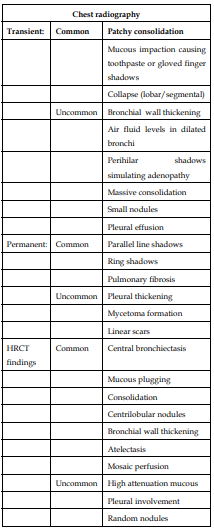
7) Radiological manifestations
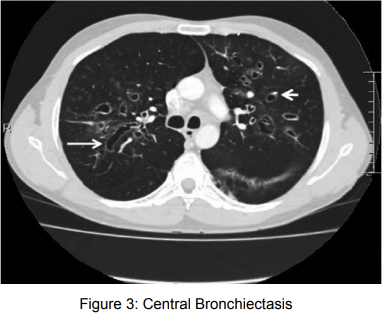
Differential Diagnosis13
1. Aspergillus hypersensitive bronchial asthma
2. Tuberculosis/CAP
3. Inflammatory pulmonary disorders – eosinophilic pneumonia, bronchocenteric
Complications13
1) Recurrent asthma exacerbations
2) Bronchiectasis
3) Pulmonary hypertension
4) Respiratory failure
5) End stage fibrotic lung disease
Treatment
The goals of treatment of ABPA according to the AAAAI Committee report on ABPA32 are
1) Control symptoms of asthma or CF2) Prevent or treat pulmonary exacerbations of ABPA
3) Reduce or remit pulmonary inflammation
4) Mitigate progression to end stage fibrotic
or cavitatory disease.
a) Oral Steroids
Oral corticosteroids is the treatment of choice for ABPA.
Steroid dosing in ABPA
Stage 1(Acute) and Stage 3(Exacerbation)33
Prednisolone 0.5mg/kg/day for 2 weeks followed by alternate days for 6-8 weeks. Once Ig declines by 1/3rd and radiological opacities cleared, tapered 2.5 to 5mg every 2 weeks. After completion of therapy, patient monitored for 2 months to assure remission.
Stage 4 (steroid dependent asthma) –
Alternate therapy with prednisolone 10- 40mg/day to maintain symptom control34
Stage 5 (Fibrotic lung disease) – management of end stage lung disease
Pulse therapy with intravenous methylpred nisolone using 10 to 20 mg/kg/day for 3 consecutive days has been shown to be useful in managing severe and sometimes life-threatening exacerbations among children with ABPA and cystic fibrosis35
b) Antifungals (azole)
Antifungals reduce the fungal load thereby decreasing the antigenic stimulus and inflammatory response and proven to have adjunctive role in steroid dependent or steroid unresponsive patients36. They are also used in initial therapy of acute and exacerbation of ABPA37. The mechanism of action is by blocking the CYP450 dependent demthylation of lanosterol, leading to the inhibition of ergosterol synthesis. Ergosterol depletion enchances fungal cell susceptibility to both oxidative and nonoxidative phagocytic damage. The most common used drug is itraconazole followed by newer azoles, voriconazole and posaconazole. Itraconazole has low oral bioavailability and high side effect profile. Dose adjustments are required for patients with mild to moderate hepatic dysfunction(Child class A and B). Monitoring of LFT is recommended at baseline, within the first 2 weeks of treatment initiation and periodically thereafter. The dosage of itraconazole recommended is 200 mg twice daily for 4 to 6 months, which is then tapered over the next 4 to 6 months. Oral itroconazole has side effect of nausea and diarrhea causes by the excipient hydroxypropyl B cyclodextrin which is used to increase the solubility of the drug. It is associated with a unique triad of hypertension, hypokalemia, and edema. Also causes negative inotropic effect avoiding administration in patients with a history of heart failure. By inhibiting steroid metabolism and thereby exacerbating adrenal suppression, itraconazole might lead to cushingoid features when used for very long durations.
Voriconazole is extended spectrum azole with good oral bioavailability and does not inhibit steroid metabolism. It is associated with visual disturbances and cutaneous phototoxicity in a minority of patients.
c) AntiIgE
Omalizumab, a monoclonal antibody against IgE has been tried and shown excellent results in patients with steroid dependent ABPA38-39.
Conclusion
All patients with uncontrolled asthma should be evaluated for allergic bronchopulmonary aspergillosis. Diagnosis of ABPA is based on clinical, immunological and radiological criteria and the management is primarily steroids with antifungals having an adjunct role.
References
1. Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest 2002; 121:1988 –1999
2. Rajan TV. The Gell-Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol 2003; 24:376 – 379
3. Geha RS, Sampson HA, Askenase PW, et al. Allergy and hypersensitivity. In: Janeway CA, Travers P, Walport M, et al. eds. Immunobiology. New York, NY: Garland, 2001; 517–556
4. Hinson, K., Moon, A., & Plummer, N. (1952). Bronchopulmonary Aspergillosis : A Review and a Report of Eight New Cases. Thorax, 7(4), 317-333
5. Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43:850-73.
6. Chauhan B, Santiago L, Kirschmann DA, Hauptfeld V, Knutsen AP, Hutcheson PS, et al. The association of HLADR alleles and T cell activation with allergic bronchopulmonary aspergillosis. J Immunol 1997;159:4072- 6.
7. Chauhan B, Santiago L, Hutcheson PS, Schwartz HJ, Spitznagel E, Castro M, et al. Evidence for the involvement of two different MHC class II regions in susceptibility or protection in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 2000; 106:723-9
8. Miller PW, Hamosh A, Macek M Jr, Greenberger PA, MacLean J, Walden SM, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in allergic bronchopulmonary aspergillosis. Am J Hum Genet 1996;59:45-51.
9. Eaton TE, Weiner Miller P, Garrett JE, Cutting GR. Cystic fibrosis transmembrane conductance regulator gene mutations: do they play a role in the aetiology of allergic bronchopulmonary aspergillosis? Clin Exp Allergy 2002;32:756-61.
10. Saxena S, Madan T, Shah A, Muralidhar K, Sarma PU. Association of polymorphisms in the collagen region of SPA2 with increased levels of total IgE antibodies and eosinophilia in patients with allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 2003;111:1001-7.
11. Kaur S, Gupta VK, Shah A, Thiel S, Sarma PU, Madan T. Elevated levels of mannan-binding lectin [corrected] (MBL) and eosinophilia in patients of bronchial asthma with allergic rhinitis and allergic bronchopulmonary aspergillosis associate with a novel intronic polymorphism in MBL. Clin Exp Immunol 2006;143:414-9.
12. Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW, Rodrigues F. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis 2008;197:618-21.
13. Agarwal, R. (2009). Allergic Bronchopulmonary Aspergillosis. Chest, 135(3), 805-826. doi: 10.1378/chest.08- 2586
14. Chan-Yeung M, Chase WH, Trapp W, et al. Allergic bron chopulmonary aspergillosis. Clinical and pathologic study of three cases. Chest 1971; 59:33–39
15. 66 Riley DJ, Mackenzie JW, Uhlman WE, et al. Allergicbronchopulmonary aspergillosis: evidence of limited tissue invasion. Am Rev Respir Dis 1975; 111:232– 23
16. Case records of the Massachusetts General Hospital.Weekly clinicopathological exercises. Case 24– 2001. A 46-year-old woman withchronic sinusitis, pulmonary nodules, and hemoptysis. N Engl J Med 2001; 345:443–449
17. Agarwal, R. (2009). Allergic Bronchopulmonary Aspergillosis. Chest, 135(3), 805-826. doi: 10.1378/chest.08- 2586
18. Rosenberg M, Patterson R, Mintzer R, et al. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med 1977; 86:405–414
19. Patterson R, Greenberger PA, Halwig JM, et al. Allergic bronchopulmonary aspergillosis: natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med 1986; 146:916– 918
20. Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43:850-73.
21. Patterson R, Greenberger PA, Radin RC, Roberts M. Allergic bronchopulmonary aspergillosis: staging as an aid to management. Ann Intern Med 1982;96:286-91.
22. Greenberger PA, Patterson R. Diagnosis and management of allergic bronchopulmonary aspergillosis. Ann Allergy 1986;56:444-8.
23. Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43:850-73.
24. Agarwal R, Gupta D, Aggarwal AN, et al. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in North India. Chest 2006; 130:442– 448
25. Ricketti AJ, Greenberger PA, Patterson R. Serum IgE as an important aid in management of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 1984; 74:68–71
26. 115 Malo JL, Hawkins R, Pepys J. Studies in chronic allergic bronchopulmonary aspergillosis: 1. Clinical and physiological findings. Thorax 1977; 32:254–261
27. McCarthy DS, Pepys J. Allergic broncho-pulmonary aspergillosis. Clinical immunology: 1. Clinical features. Clin Allergy 1971; 1:261–286
28. Nichols D, Dopico GA, Braun S, et al. Acute and chronic pulmonary function changes in allergic bronchopulmonary aspergillosis. Am J Med 1979; 67:631–637
29. Panjabi C, Shah A. Lung functions in allergic bronchopulmonary aspergillosis. Respirology 2006;11:A38.
30. Longbottom JL, Pepys J. Pulmonary aspergillosis: diagnostic and immunological significance of antigens and C-substance in Aspergillus fumigatus. J Pathol Bacteriol1964; 88:141–151
31. Vlahakis NE, Aksamit TR. Diagnosis and treatment of allergic broncho pulmonary aspergillosis. Mayo Clin Proc 2001; 76:930–938
32. Greenberger PA, Bush RK, Demain JG, Luong A, Slavin RG, Knutsen AP. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2014;2:703-8.
33. Greenberger PA. Allergic bronchopulmonary aspergillosis.J Allergy Clin Immunol 2002; 110:685– 692
34. Shah, A., & Panjabi, C. (2016). Allergic Bronchopulmonary Aspergillosis: A Perplexing Clinical Entity. Allergy, Asthma & Immunology Research, 8(4), 282. doi: 10.4168/aair.2016.8.4.282
35. Thomson JM, Wesley A, Byrnes CA, Nixon GM. Pulse intravenous methylprednisolone for resistant allergic bronchopulmonary aspergillosis in cystic fibrosis. Pediatr Pulmonol 2006;41:164-70.
36. Leon EE, Craig TJ. Antifungals in the treatment of allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol 1999; 82:511-6.
37. Ullmann, A., Aguado, J et al. (2018). Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clinical Microbiology And Infection, 24, e1-e38
38. Tillie-Leblond I, Germaud P, Leroyer C, Tétu L, Girard F, Devouassoux G, et al. Allergic bronchopulmonary aspergillosis and omalizumab. Allergy 2011;66:1254-6.
39. Collins J, Devos G, Hudes G, Rosenstreich D. Allergic bronchopulmonary aspergillosis treated successfully for one year with omalizumab. J Asthma Allergy 2012;5:65-70.





