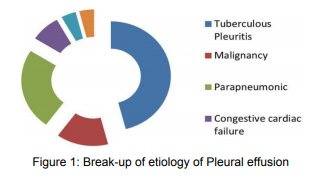
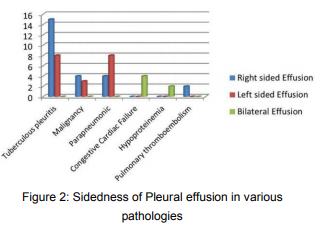
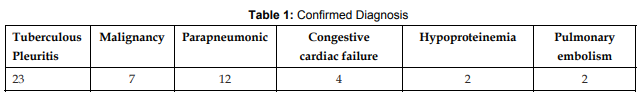
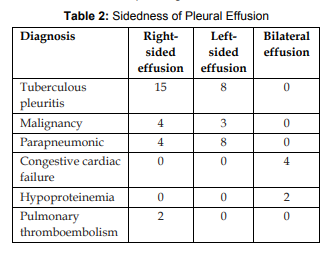
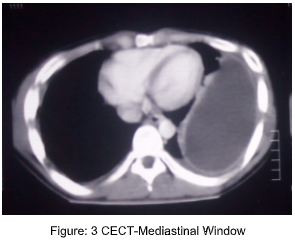
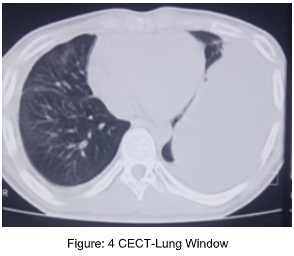
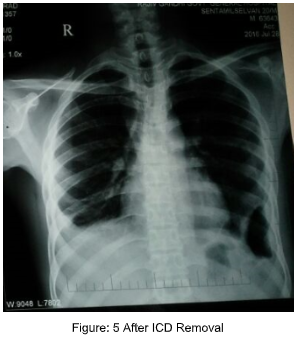
References
1. Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest 2002; 121:1988 –1999
2. Rajan TV. The Gell-Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol 2003; 24:376 – 379
3. Geha RS, Sampson HA, Askenase PW, et al. Allergy and hypersensitivity. In: Janeway CA, Travers P, Walport M, et al. eds. Immunobiology. New York, NY: Garland, 2001; 517–556
4. Hinson, K., Moon, A., & Plummer, N. (1952). Bronchopulmonary Aspergillosis : A Review and a Report of Eight New Cases. Thorax, 7(4), 317-333
5. Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43:850-73.
6. Chauhan B, Santiago L, Kirschmann DA, Hauptfeld V, Knutsen AP, Hutcheson PS, et al. The association of HLADR alleles and T cell activation with allergic bronchopulmonary aspergillosis. J Immunol 1997;159:4072- 6.
7. Chauhan B, Santiago L, Hutcheson PS, Schwartz HJ, Spitznagel E, Castro M, et al. Evidence for the involvement of two different MHC class II regions in susceptibility or protection in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 2000; 106:723-9
8. Miller PW, Hamosh A, Macek M Jr, Greenberger PA, MacLean J, Walden SM, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in allergic bronchopulmonary aspergillosis. Am J Hum Genet 1996;59:45-51.
9. Eaton TE, Weiner Miller P, Garrett JE, Cutting GR. Cystic fibrosis transmembrane conductance regulator gene mutations: do they play a role in the aetiology of allergic bronchopulmonary aspergillosis? Clin Exp Allergy 2002;32:756-61.
10. Saxena S, Madan T, Shah A, Muralidhar K, Sarma PU. Association of polymorphisms in the collagen region of SPA2 with increased levels of total IgE antibodies and eosinophilia in patients with allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 2003;111:1001-7.
11. Kaur S, Gupta VK, Shah A, Thiel S, Sarma PU, Madan T. Elevated levels of mannan-binding lectin [corrected] (MBL) and eosinophilia in patients of bronchial asthma with allergic rhinitis and allergic bronchopulmonary aspergillosis associate with a novel intronic polymorphism in MBL. Clin Exp Immunol 2006;143:414-9.
12. Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW, Rodrigues F. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis 2008;197:618-21.
13. Agarwal, R. (2009). Allergic Bronchopulmonary Aspergillosis. Chest, 135(3), 805-826. doi: 10.1378/chest.08- 2586
14. Chan-Yeung M, Chase WH, Trapp W, et al. Allergic bron chopulmonary aspergillosis. Clinical and pathologic study of three cases. Chest 1971; 59:33–39
15. 66 Riley DJ, Mackenzie JW, Uhlman WE, et al. Allergicbronchopulmonary aspergillosis: evidence of limited tissue invasion. Am Rev Respir Dis 1975; 111:232– 23
16. Case records of the Massachusetts General Hospital.Weekly clinicopathological exercises. Case 24– 2001. A 46-year-old woman withchronic sinusitis, pulmonary nodules, and hemoptysis. N Engl J Med 2001; 345:443–449
17. Agarwal, R. (2009). Allergic Bronchopulmonary Aspergillosis. Chest, 135(3), 805-826. doi: 10.1378/chest.08- 2586
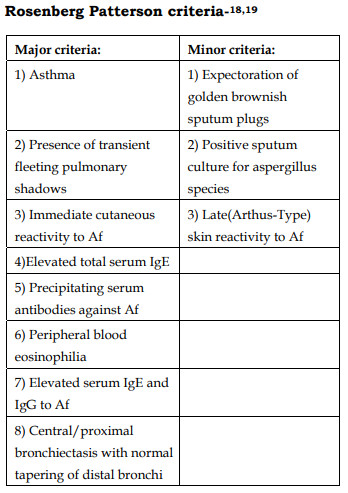
18. Rosenberg M, Patterson R, Mintzer R, et al. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med 1977; 86:405–414
19. Patterson R, Greenberger PA, Halwig JM, et al. Allergic bronchopulmonary aspergillosis: natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med 1986; 146:916– 918
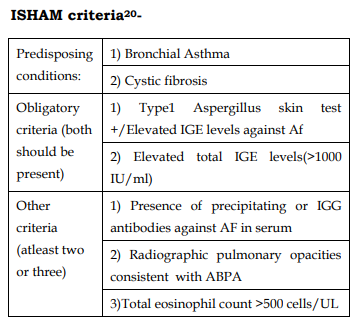
20. Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43:850-73.
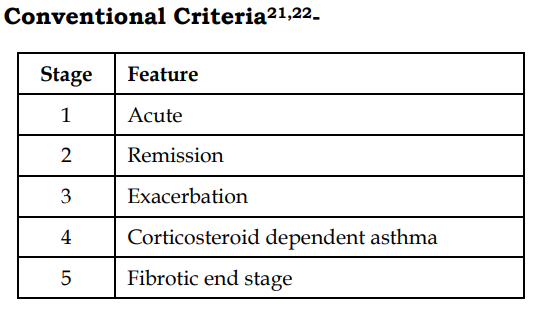
21. Patterson R, Greenberger PA, Radin RC, Roberts M. Allergic bronchopulmonary aspergillosis: staging as an aid to management. Ann Intern Med 1982;96:286-91.
22. Greenberger PA, Patterson R. Diagnosis and management of allergic bronchopulmonary aspergillosis. Ann Allergy 1986;56:444-8.
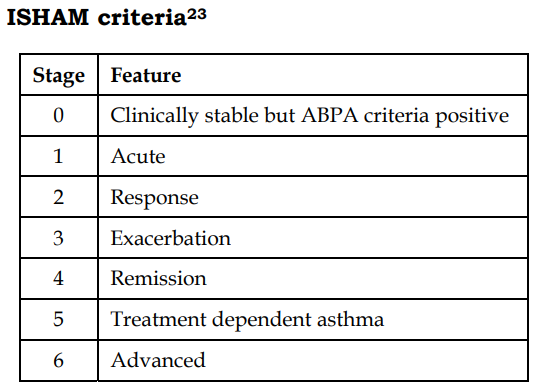
23. Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43:850-73.
24. Agarwal R, Gupta D, Aggarwal AN, et al. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in North India. Chest 2006; 130:442– 448
25. Ricketti AJ, Greenberger PA, Patterson R. Serum IgE as an important aid in management of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 1984; 74:68–71
26. 115 Malo JL, Hawkins R, Pepys J. Studies in chronic allergic bronchopulmonary aspergillosis: 1. Clinical and physiological findings. Thorax 1977; 32:254–261
27. McCarthy DS, Pepys J. Allergic broncho-pulmonary aspergillosis. Clinical immunology: 1. Clinical features. Clin Allergy 1971; 1:261–286
28. Nichols D, Dopico GA, Braun S, et al. Acute and chronic pulmonary function changes in allergic bronchopulmonary aspergillosis. Am J Med 1979; 67:631–637
29. Panjabi C, Shah A. Lung functions in allergic bronchopulmonary aspergillosis. Respirology 2006;11:A38.
30. Longbottom JL, Pepys J. Pulmonary aspergillosis: diagnostic and immunological significance of antigens and C-substance in Aspergillus fumigatus. J Pathol Bacteriol1964; 88:141–151
31. Vlahakis NE, Aksamit TR. Diagnosis and treatment of allergic broncho pulmonary aspergillosis. Mayo Clin Proc 2001; 76:930–938
32. Greenberger PA, Bush RK, Demain JG, Luong A, Slavin RG, Knutsen AP. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2014;2:703-8.
33. Greenberger PA. Allergic bronchopulmonary aspergillosis.J Allergy Clin Immunol 2002; 110:685– 692
34. Shah, A., & Panjabi, C. (2016). Allergic Bronchopulmonary Aspergillosis: A Perplexing Clinical Entity. Allergy, Asthma & Immunology Research, 8(4), 282. doi: 10.4168/aair.2016.8.4.282
35. Thomson JM, Wesley A, Byrnes CA, Nixon GM. Pulse intravenous methylprednisolone for resistant allergic bronchopulmonary aspergillosis in cystic fibrosis. Pediatr Pulmonol 2006;41:164-70.
36. Leon EE, Craig TJ. Antifungals in the treatment of allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol 1999; 82:511-6.
37. Ullmann, A., Aguado, J et al. (2018). Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clinical Microbiology And Infection, 24, e1-e38
38. Tillie-Leblond I, Germaud P, Leroyer C, Tétu L, Girard F, Devouassoux G, et al. Allergic bronchopulmonary aspergillosis and omalizumab. Allergy 2011;66:1254-6.
39. Collins J, Devos G, Hudes G, Rosenstreich D. Allergic bronchopulmonary aspergillosis treated successfully for one year with omalizumab. J Asthma Allergy 2012;5:65-70.